-
PDF
-
Views
-
Cite
Cite
Margherita Russo, Antonino Naro, Antonino Leo, Edoardo Sessa, Giangaetano D’Aleo, Placido Bramanti, Rocco Salvatore Calabrò, Evaluating Sativex ® in Neuropathic Pain Management: A Clinical and Neurophysiological Assessment in Multiple Sclerosis , Pain Medicine, Volume 17, Issue 6, June 2016, Pages 1145–1154, https://doi.org/10.1093/pm/pnv080
Close - Share Icon Share
Abstract
Objective . The aim of our study was to better investigate the role of Sativex ® in improving pain in multiple sclerosis (MS) patients by means of either clinical or neurophysiological assessment.
Setting. Pain is a common symptom of MS, affecting up to 70% of patients. Pain treatment is often unsatisfactory, although emerging drugs (including cannabinoids) are giving encouraging results. Clinical pain assessment in MS is very difficult, and more objective tools are necessary to better quantify this symptom and its potential response to the treatments.
Subjects and Methods. We enrolled 20 MS patients (10 with and 10 without neuropathic pain), who underwent a specific clinical (such as visual analog scale) and neurophysiological assessment (by means of laser-evoked potentials and transcranial magnetic stimulation), before and after 4 weeks of Sativex administration.
Results One month of drug administration in MS patients with neuropathic pain successfully reduced pain rating and improved quality of life. Interestingly, such effects were paralleled by an increase of fronto-central γ-band oscillation and of pain-motor integration strength.
Conclusions. Our data suggest that Sativex may be effective in improving MS-related neuropathic pain, maybe through its action on specific cortical pathways.
Introduction
Multiple sclerosis (MS) is a progressive, inflammatory, autoimmune disease of the central nervous system, characterized by demyelination and variable axonal loss. MS represents one of the most common causes of neurological disability in young people, as it may cause a wide range of chronic symptoms, including spasticity, spasms, fatigue, bladder dysfunction, and pain. Pain may be defined as an “unpleasant sensory experience associated with actual or potential tissue damage or described in terms of such damage.” It is a common symptom of MS, affecting up to 70% of patients, but there are no clear markers of susceptibility to pain development. MS patients may experience many pain syndromes, including i) continuous and intermittent central neuropathic pain (i.e., trigeminal neuralgia, Lhermitte sign, glosso-pharyngeal neuralgia); 2) musculoskeletal pain (i.e., painful tonic spasms, pain secondary to spasticity, pain related to being wheelchair-bound); and 3) mixed neuropathic/non-neuropathic pain (i.e., headache) [ 1 ]. However, the most common type is the central neuropathic pain, often described as burning, dragging, aching, or paroxysmal neuralgias. Noteworthy, central neuropathic pain excludes other types of pain, including pain associated with spasticity and rigidity, which instead depends on the activation of the muscle nociceptive afferents [ 2 ]. Nevertheless, the pathophysiology of central neuropathic pain is still partially understood, so that its clinical assessment and treatment are challenging [ 2 ]. The current therapeutic options are limited to antiepileptics (with well-known tolerability problems), tricyclic antidepressants, short-term non-steroidal anti-inflammatory drugs, and analgesics [ 1 , 3–4 ]. Notably, growing evidence suggests that cannabinoids, such as dronabinol, cannabidiol (CBD), nabilone, and nabiximols (Sativex ® , GW Pharma, Histon, Cambridge, UK) may be useful in the treatment of pain and spasticity in many neurological diseases, including MS. Indeed, a recent meta-analysis found a statistically significant efficacy of cannabinoids in MS pain relief [ 5 ], although there are conflicting reports in the literature [ 6–7 ].
Sativex is an oromucosal spray formulation (recently approved in Italy for the treatment of moderate to severe spasticity in MS), derived from cloned Cannabis sativa L. plant, containing delta-9-tetrahydrocannabinol (THC) and CBD at an approximately 1:1 fixed ratio. THC is the main active substance, acts as a partial agonist at human cannabinoid CB1 and CB2 receptors, and may modulate the excitatory (glutamate) and inhibitory (gamma-aminobutyric acid) neurotransmission.
On the other side, CBD may antagonize some effects of THC, including the anxiogenic and psychoactive. Nevertheless, CBD may also antagonize some potentially desirable THC effects; for example, appetite enhancement [ 8–10 ]. Interestingly, recent studies strongly suggested that oromucosal-spray cannabinoids are also effective in pain relief, as suggested by subjective pain rating reduction, with mild side-effects [ 6 , 11 , 13–14 ]. Nevertheless, other reports have described a lack of Sativex effectiveness, thus suggesting a more cautious and precise prescription of this medication [ 6 , 12 ].
Finding the proper treatment for MS pain relief is mostly related to the right assessment, which is often challenging because of the complexity of clinical manifestations and pathophysiology, also taking into account the psychological and social factors. As the most commonly used clinical scales for pain assessment (including verbal category, numeric, and visual analog [VAS] scales) may be biased by patient and clinician subjective evaluation [ 15 ], more objective tools are necessary to better define and quantify pain, and select the most adequate treatment. To this end, cortical laser-evoked potentials (LEPs) have been shown to be useful for the evaluation of the integrity of the nociception afferent pathways [ 16 ], so to distinguish neuropathic (e.g., LEP latency increase) from non-neuropathic pain [ 17 , 18 ]. Noteworthy, although LEP alterations may indicate a nociceptive pathway dysfunction, they fail in reflecting the subjective pain perception, as LEP characteristics strongly depend on attentive level, stimulus’ saliency (nociceptive or not), and repetition [ 19 ].
On the other side, the estimation of the cortical LEP γ-band power has been recently suggested to be positively correlated to the subjective perception of pain in terms of cortical gating mechanism, without any attention and stimulus repetition influence [ 20 , 21 ]. Therefore, the γ-band power estimation could be a useful marker of subjective pain perception. To this end, it has been also shown that relevant painful stimuli may at an unaware level modify motor cortex excitability; namely, pain motor integration (PMI) [ 22 ], in order to allow subcortical defensive reflex elicitation, purposeful behavior actuation, and cortical pain-gating phenomena. Therefore, the aim of our study was to investigate the role of Sativex in improving pain in MS patients by means of a novel neurophysiological assessment, including LEP, PMI, and γ-band power estimation, besides the standard clinical evaluation.
Methods
Subjects’ Enrollment
Of the 52 MS patients attending our research institute, who met the regional (i.e., Sicily) inclusion criteria for Sativex administration, we selected those complaining of central neuropathic pain (pain-MS) (27 patients) according to the douleur neuropathique 4 questionnaire (DN4, ≥4) [ 23 ]. Patients were then selected according to the following inclusion criteria: diagnosis of definite MS for 6 months, based on Polman criteria, [ 24 , 25 ] age ≥18 years, absence of clinical or neuroradiological relapses for at least 6 months prior to study entry, an Expanded Disability Status Scale (EDSS) total score ≥3.5, right-handedness, right side being the most affected, no history of psychosis, no safety contraindication for TMS/laser procedures. Patients with severe pain from other concomitant conditions were excluded. Thus, 10 of the 27 eligible patients entered the study. These patients were compared with 10 MS pain-free individuals (no pain-MS) also treated with Sativex. Clinical characteristics of patients are reported in Table 1 . All patients were taking antispastics, baclofen being the most common. Only 40% of patients occasionally took other medications, mainly analgesics, for pain. The experiment was approved by the local ethics committee and all subjects gave their written informed consent, according to the declaration of Helsinki.
reports the clinical characteristics at baseline (PRE) of both groups, at individual and group (mean ± standard deviation -sd) level. There were no significant differences between the two groups regarding age, disease duration (dd), and NRS, whereas VAS and DN4 scores were above the cut-off value (≥4) in the pain-MS patients.
| . | gender . | age . | EDSS . | dd . | NRS* . | MAS . | pt . | VAS† . | DN4 . |
|---|---|---|---|---|---|---|---|---|---|
| p-MS | F | 53 | 6,5 | 48 | 8 | 2 | BP | 5 | 7 |
| F | 51 | 6,5 | 165 | 8 | 3 | TN | 5 | 7 | |
| M | 52 | 7 | 96 | 8 | 4 | BP | 6 | 7 | |
| F | 42 | 5,0 | 276 | 9 | 2 | BP | 7 | 7 | |
| M | 49 | 7,5 | 398 | 8 | 4 | LH | 7 | 9 | |
| M | 45 | 6,5 | 144 | 10 | 3 | BP | 7 | 7 | |
| F | 43 | 7,5 | 240 | 8 | 4 | BP | 7 | 6 | |
| F | 53 | 7,5 | 279 | 8 | 4 | BP | 7 | 5 | |
| F | 48 | 4,0 | 332 | 9 | 3 | LH | 9 | 9 | |
| M | 52 | 6,5 | 361 | 8 | 3 | TN | 9 | 10 | |
| mean ± sd | 49 ± 4 | 6 | 234 ± 117 | 8 ± 1 | 3 | 7 ± 1 | 7 ± 2 | ||
| np-MS | M | 43 | 6,5 | 300 | 8 | 4 | 0 | 0 | |
| F | 51 | 7,0 | 240 | 9 | 4 | 0 | 0 | ||
| M | 46 | 6,5 | 48 | 9 | 2 | BP | 1 | 1 | |
| F | 40 | 7,5 | 300 | 8 | 4 | 0 | 0 | ||
| F | 53 | 7,0 | 420 | 9 | 3 | 0 | 1 | ||
| M | 38 | 7,5 | 114 | 8 | 4 | BP | 2 | 1 | |
| F | 37 | 7,0 | 120 | 8 | 4 | BP | 1 | 2 | |
| M | 37 | 7,0 | 160 | 9 | 4 | BP | 2 | 1 | |
| F | 39 | 7,3 | 168 | 9 | 3 | 0 | 0 | ||
| F | 48 | 7,5 | 140 | 8 | 4 | 0 | 0 | ||
| mean ± sd | 43 ± 6 | 7 | 201 ± 112 | 9 ± 1 | 4 | 1 ± 1 | 2 ± 1 | ||
| . | gender . | age . | EDSS . | dd . | NRS* . | MAS . | pt . | VAS† . | DN4 . |
|---|---|---|---|---|---|---|---|---|---|
| p-MS | F | 53 | 6,5 | 48 | 8 | 2 | BP | 5 | 7 |
| F | 51 | 6,5 | 165 | 8 | 3 | TN | 5 | 7 | |
| M | 52 | 7 | 96 | 8 | 4 | BP | 6 | 7 | |
| F | 42 | 5,0 | 276 | 9 | 2 | BP | 7 | 7 | |
| M | 49 | 7,5 | 398 | 8 | 4 | LH | 7 | 9 | |
| M | 45 | 6,5 | 144 | 10 | 3 | BP | 7 | 7 | |
| F | 43 | 7,5 | 240 | 8 | 4 | BP | 7 | 6 | |
| F | 53 | 7,5 | 279 | 8 | 4 | BP | 7 | 5 | |
| F | 48 | 4,0 | 332 | 9 | 3 | LH | 9 | 9 | |
| M | 52 | 6,5 | 361 | 8 | 3 | TN | 9 | 10 | |
| mean ± sd | 49 ± 4 | 6 | 234 ± 117 | 8 ± 1 | 3 | 7 ± 1 | 7 ± 2 | ||
| np-MS | M | 43 | 6,5 | 300 | 8 | 4 | 0 | 0 | |
| F | 51 | 7,0 | 240 | 9 | 4 | 0 | 0 | ||
| M | 46 | 6,5 | 48 | 9 | 2 | BP | 1 | 1 | |
| F | 40 | 7,5 | 300 | 8 | 4 | 0 | 0 | ||
| F | 53 | 7,0 | 420 | 9 | 3 | 0 | 1 | ||
| M | 38 | 7,5 | 114 | 8 | 4 | BP | 2 | 1 | |
| F | 37 | 7,0 | 120 | 8 | 4 | BP | 1 | 2 | |
| M | 37 | 7,0 | 160 | 9 | 4 | BP | 2 | 1 | |
| F | 39 | 7,3 | 168 | 9 | 3 | 0 | 0 | ||
| F | 48 | 7,5 | 140 | 8 | 4 | 0 | 0 | ||
| mean ± sd | 43 ± 6 | 7 | 201 ± 112 | 9 ± 1 | 4 | 1 ± 1 | 2 ± 1 | ||
EDSS: expanded disability status scale; dd: disease duration; NRS: numerical rating scale; MAS: modified Ashworth scale; VAS: visual analog scale; DN4 douleur neuropathique 4 questionnaire; pt: pain type (BP: burning pain; TN: trigeminal neuralgia; LH: Lhermitte sign).
reports the clinical characteristics at baseline (PRE) of both groups, at individual and group (mean ± standard deviation -sd) level. There were no significant differences between the two groups regarding age, disease duration (dd), and NRS, whereas VAS and DN4 scores were above the cut-off value (≥4) in the pain-MS patients.
| . | gender . | age . | EDSS . | dd . | NRS* . | MAS . | pt . | VAS† . | DN4 . |
|---|---|---|---|---|---|---|---|---|---|
| p-MS | F | 53 | 6,5 | 48 | 8 | 2 | BP | 5 | 7 |
| F | 51 | 6,5 | 165 | 8 | 3 | TN | 5 | 7 | |
| M | 52 | 7 | 96 | 8 | 4 | BP | 6 | 7 | |
| F | 42 | 5,0 | 276 | 9 | 2 | BP | 7 | 7 | |
| M | 49 | 7,5 | 398 | 8 | 4 | LH | 7 | 9 | |
| M | 45 | 6,5 | 144 | 10 | 3 | BP | 7 | 7 | |
| F | 43 | 7,5 | 240 | 8 | 4 | BP | 7 | 6 | |
| F | 53 | 7,5 | 279 | 8 | 4 | BP | 7 | 5 | |
| F | 48 | 4,0 | 332 | 9 | 3 | LH | 9 | 9 | |
| M | 52 | 6,5 | 361 | 8 | 3 | TN | 9 | 10 | |
| mean ± sd | 49 ± 4 | 6 | 234 ± 117 | 8 ± 1 | 3 | 7 ± 1 | 7 ± 2 | ||
| np-MS | M | 43 | 6,5 | 300 | 8 | 4 | 0 | 0 | |
| F | 51 | 7,0 | 240 | 9 | 4 | 0 | 0 | ||
| M | 46 | 6,5 | 48 | 9 | 2 | BP | 1 | 1 | |
| F | 40 | 7,5 | 300 | 8 | 4 | 0 | 0 | ||
| F | 53 | 7,0 | 420 | 9 | 3 | 0 | 1 | ||
| M | 38 | 7,5 | 114 | 8 | 4 | BP | 2 | 1 | |
| F | 37 | 7,0 | 120 | 8 | 4 | BP | 1 | 2 | |
| M | 37 | 7,0 | 160 | 9 | 4 | BP | 2 | 1 | |
| F | 39 | 7,3 | 168 | 9 | 3 | 0 | 0 | ||
| F | 48 | 7,5 | 140 | 8 | 4 | 0 | 0 | ||
| mean ± sd | 43 ± 6 | 7 | 201 ± 112 | 9 ± 1 | 4 | 1 ± 1 | 2 ± 1 | ||
| . | gender . | age . | EDSS . | dd . | NRS* . | MAS . | pt . | VAS† . | DN4 . |
|---|---|---|---|---|---|---|---|---|---|
| p-MS | F | 53 | 6,5 | 48 | 8 | 2 | BP | 5 | 7 |
| F | 51 | 6,5 | 165 | 8 | 3 | TN | 5 | 7 | |
| M | 52 | 7 | 96 | 8 | 4 | BP | 6 | 7 | |
| F | 42 | 5,0 | 276 | 9 | 2 | BP | 7 | 7 | |
| M | 49 | 7,5 | 398 | 8 | 4 | LH | 7 | 9 | |
| M | 45 | 6,5 | 144 | 10 | 3 | BP | 7 | 7 | |
| F | 43 | 7,5 | 240 | 8 | 4 | BP | 7 | 6 | |
| F | 53 | 7,5 | 279 | 8 | 4 | BP | 7 | 5 | |
| F | 48 | 4,0 | 332 | 9 | 3 | LH | 9 | 9 | |
| M | 52 | 6,5 | 361 | 8 | 3 | TN | 9 | 10 | |
| mean ± sd | 49 ± 4 | 6 | 234 ± 117 | 8 ± 1 | 3 | 7 ± 1 | 7 ± 2 | ||
| np-MS | M | 43 | 6,5 | 300 | 8 | 4 | 0 | 0 | |
| F | 51 | 7,0 | 240 | 9 | 4 | 0 | 0 | ||
| M | 46 | 6,5 | 48 | 9 | 2 | BP | 1 | 1 | |
| F | 40 | 7,5 | 300 | 8 | 4 | 0 | 0 | ||
| F | 53 | 7,0 | 420 | 9 | 3 | 0 | 1 | ||
| M | 38 | 7,5 | 114 | 8 | 4 | BP | 2 | 1 | |
| F | 37 | 7,0 | 120 | 8 | 4 | BP | 1 | 2 | |
| M | 37 | 7,0 | 160 | 9 | 4 | BP | 2 | 1 | |
| F | 39 | 7,3 | 168 | 9 | 3 | 0 | 0 | ||
| F | 48 | 7,5 | 140 | 8 | 4 | 0 | 0 | ||
| mean ± sd | 43 ± 6 | 7 | 201 ± 112 | 9 ± 1 | 4 | 1 ± 1 | 2 ± 1 | ||
EDSS: expanded disability status scale; dd: disease duration; NRS: numerical rating scale; MAS: modified Ashworth scale; VAS: visual analog scale; DN4 douleur neuropathique 4 questionnaire; pt: pain type (BP: burning pain; TN: trigeminal neuralgia; LH: Lhermitte sign).
Study Drug
Patients received cannabis-based medicine extract (Sativex) presented in a pump action sublingual spray. Sativex is composed of cannabis plant extract, containing THC (27 mg/mL) and CBD (25 mg/mL), with ethanol/propylene glycol (50:50) excipient. Each actuation delivers 100 µL of spray, containing THC 2.7 mg and CBD 2.5 mg. The mean number of sprays daily administrated was eight.
Clinical-Neurophysiological Examination
All patients underwent an accurate neurological examination, and the EDSS was performed to assess MS disability. In order to ascertain the causal or casual association between pain and the disease, all MS patients completed the DN4 questionnaire for neuropathic pain. Indeed, a definite diagnosis of central neuropathic pain was supported by the patient history and the clinical examination, including the DN4 questionnaire (≥4), which evaluates positive and negative sensory signs with a logical neuro-anatomical distribution. In addition, patients were asked to report pain (burning, dragging, aching, or paroxysmal neuralgias) experienced during the last month through a VAS [ 26 ], thus rating pain intensity on an 11-point numerical scale ranging from 0 (no disturbance) to 10 (worst possible disturbance). On the other hand, subjects rated the spasticity by means of a numerical rating scale (NRS) [ 27 ], whereas the clinicians quantified it through the Modified Ashworth scale (MAS) [ 28 ]. Moreover, we administered the MSQoL-54 scale, taking into consideration the impact of pain and spasticity on quality of life [ 29 ]. The following electrophysiological parameters from the right hand, including resting motor threshold (RMT) and motor evoked potentials (MEP) amplitude from the abductor pollicis brevis muscle (APB), PMI, latency and amplitude of N2P2 complex of LEP, RIII-wave threshold, were also detected. Finally, we estimated the γ-band power within frontal and centro-parietal areas. All clinical and neurophysiological parameters were measured as absolute values at baseline (PRE) and after 1 month (POST) of continuous treatment.
TMS Set-Up and Single-Pulse Measures
MEPs were obtained through magnetic monophasic stimuli delivered by a high-power Magstim 200 stimulator (Magstim TM , Whitland, Dyfed, UK). The coil was placed tangentially to the scalp with the handle pointing backwards and laterally, at a 45-degree angle to the sagittal plane, approximately perpendicular to the central sulcus of the left hemisphere, on the optimal site on the scalp to get the wider MEP amplitude (motor hot-spot), from the APB muscle of right hand. The rise time of the magnetic monophasic stimulus was about 100 µs with a to-zero of about 800 µs. The current flowed in handle direction during the rise-time of the magnetic field, thus with a posterior-anterior direction. We preliminarily evaluated the RMT, defined as the smallest stimulus intensity able to evoke a peak-to-peak MEP of 50 µV in rest APB, in at least five of 10 tracks consecutively [ 30 ]. Then, we applied an intensity of stimulation of 120% of rMT. Electromyographic activity was recorded through Ag-AgCl surface electrodes applied to APB using a classic muscle belly-tendon montage. Signals were amplified and filtered (from 32 Hz to 1 KHz) via a Digitimer TM D150 Amplifier (Digitimer TM Ltd., Welwyn Garden City, Herts, UK), and stored using a sampling frequency of 10 KHz on a personal computer for off-line analysis (Signal Software, Cambridge Electronic Design, Cambridge, UK). All data are given as mean or percentage difference in comparison to PRE values ± standard error (se).
Paired Laser-TMS Pulses
PMI was studied by delivering pairs of laser-magnetic stimuli, according to Valeriani and coworkers [ 18 ]. Each pair of stimuli was constituted by a laser conditioning stimulus, applied on skin dorsum of the right hand, at 150% of sensory threshold in healthy subject [ 31 , 32 ], and the test stimulus, delivered after the conditioning one, at the same intensity for MEP elicitation, applied on the hot-spot for the APB. The magnetic stimulation set-up was the same used for MEP elicitation. The interstimulus interval was individually set according to N1 latency +50 ms [ 33 ]. The relative change in MEP amplitude induced by the laser stimulus was taken as a measure of PMI strength. The mean amplitude of 10 conditioned MEP was expressed as percentage of the mean amplitude of the unconditioned one. By means of an electric stimulator (Digitimer D-160 stimulator), we measured the threshold of the rectified RIII response evoked from the flexor radialis carpi (FRC) of the right forearm, through the stimulation of the radial superficial nerve above the wrist. We delivered five rectangular pulses with a duration of 1 ms each and an interval of 1 ms [ 34 ] and a train of five electrical pulses delivered at a stimulus frequency of 300 Hz. The train stimulation was delivered every 10 s, in order to avoid habituation [ 34 ].
Laser-Evoked Potentials
Participants were seated in a comfortable chair and wore protective goggles. They were asked to focus on the stimulus, relax their muscles, keep their eyes opened, and gaze slightly downward. Acoustic isolation was ensured using earplugs and headphones. LEPs were recorded after skin stimulation at dorsum of the right hand by using a neodymium:yttrium-aluminium-perovskite (Nd:YAP) laser device (wavelength 1.34 µm, pulse duration 2–20 ms, maximum energy 7 J) under fiber-optic guidance (EL.EN, Florence, Italy). Stimulus intensity was set at 2.75 J, with a spot diameter of 5 mm and pulse duration of 10 ms, corresponding to an energy transfer of 21.54 J/cm. This intensity was ∼150% of pain sensory level in healthy subjects (corresponding to a pin-prick sensation followed by a heat one in at least three on five consecutive stimuli, and to a VAS of 4/10). The delivery frequency was 0.1 Hz, slightly shifting the stimulation point in order to avoid stimuli habituation and skin lesions. The handle of the stimulator was held perpendicularly to skin surface. This set-up was used during the entire session, in order to activate selectively the cutaneous nociceptive termination related to Aδ-fibers. LEPs were recorded by seven silver disc electrodes placed on the scalp, according to the international 10–20 system (Fz, Cz, Pz, C3, C4, T3, and T4). The reference electrode was placed on the nose and the ground on the forehead (Fpz). Eye movements and blinks were monitored by an electrode above the right eyebrow. Electrode impedance was kept below 5 kΩ. Signals were recorded, amplified, and filtered (bandwidth 0.5–50 Hz) with a Digitimer D360 (Digitimer Ltd), acquired at a sampling rate of 5 kHz through a 1401 plus A/D laboratory interface (Cambridge Electronic Design), and stored on a personal computer for off-line analysis (Signal Software; Cambridge Electronic Design). The time-analysis was set at 1000 ms. We averaged two non-consecutive trials of 15 LEPs, with a break of two minutes between each trial. Peak latency (ms) and peak-to-peak amplitude (µV) of N2-P2 complex were measured from vertex electrode (Cz). Peak latency of N1 response was calculated from T3 electrode, in order to set individually the adequate interstimulus interval concerning PMI assessment.
γ-Band Power Estimation
We applied four laser-stimulus trains (E 1 , E 2 , E 3 , E 4 ), employing the aforementioned laser stimulation set-up. Each train consisted of three stimuli (S 1 , S 2 , and S 3 ), at a frequency of 1 Hz and at the same intensity, in analogy to a previous study [ 31 , 35 ]. Four different energies were used (from threshold -E T - up to +1.5 J, with 0.5 J steps: E 1 -E T -, E 2 -E T+0.5J -, E 3 -E T+1J -, E 4 -E T+1.5J ). The inter-train interval was 30 s. Participants were blinded on laser stimulation procedure and were asked to verbally rate the intensity of the pricking sensation elicited by each laser stimulus of the train, using a pain NRS (ranging from zero to 10, where zero was defined as “no pain” and 10 as “pain as bad as it can be”). Twenty trains at each of the four energies (E 1 −E 4 ) were delivered, in random order, for a total of 80 trains per session. EEG epochs were extracted using a window ranging from 1 s before the onset of S 1 to 1 s after the onset of S 3 of each train (total epoch duration: 4 s) and baseline corrected using the interval ranging from −1 to the onset of S 1 (0 sec.). Trials contaminated by eye-blinks and movements were corrected using an independent component analysis algorithm [ 36 ]. EEG epochs were then visually inspected and trials contaminated by artifacts due to gross movements were removed. After artifacts rejection, EEG epochs were classified in four categories according to the intensity of the painful percept elicited by the E applied. This was achieved by rescaling the ratings of each participant between zero and 100 (being zero the lowest pain rating and 100 the highest one). Therefore, trials were classified in four categories for each participant, according to intensity of perception (I) (I 1 :<25, I 2 : from 26 to 50; I 3 : from 51 to 75; I 4 : from 76 up to 100%). Trials in each category were averaged together, thus obtaining four averaged waveforms for each participant. Hence, we quantified the absolute power applying a Fast Fourier Transform (Hamming window, frequency resolution 0.25 Hz) [ 37 ]. The mean γ-band (75-120 Hz) power was obtained by averaging single power values. The γ-band power temporal percent variations were calculated within each electrode in analogy to the Pfurtscheller formula [ 37 ].
Statistical Analysis
The Wilcoxon signed ranks test on the baseline/post-treatment (PRE-T 30 ) treatment scores for the different clinical outcome measures (EDDS, VAS, MAS, NRS, MSQoL) were carried out in both groups. The α-level for significance was set at P < 0.05. The Bonferroni correction was used in multiple comparisons. Data were expressed as mean or percentage ± standard deviation. The effects of the treatment on RMT, peak-to-peak MEP amplitude, PMI strength, RIII threshold, γ-band power, peak latency, and peak-to-peak amplitude of LEP N2-P2 complex were evaluated through separate repeated-measure analyses of variance (rmANOVA). For each dependent variable, we computed a two-way rmANOVA with time (two levels: PRE and T 30 ) as within-subject factor, and group (two levels: pain-MS and no pain-MS) as between-subject factor. For γ-band power estimation we added the within-subject factors E (four levels: E 1 , E 2 , E 3 , and E 4 ), I (four levels: I 1 , I 2 , I 3 , and I 4 ), and electrode (seven levels: Fz, Cz, Pz, C3, C4, T3, and T4). Conditional on a significant F-value, post-hoc paired-sample t- tests were performed to explore the strength of main effects and the patterns of interaction between experimental factors. When the effect of the factor E was significant, we performed a post-hoc analysis using a paired-sample t -test in order to compare the γ-band power elicited by each S. Whether a significant E*I interaction, the correlation between I and γ-band power was assessed in an attempt to find a stimulus repetition effect. The Greenhouse-Geisser method was used if necessary to correct for non-sphericity. All data are given as means (clinical parameters) or percent (electrophysiological ones), ±se. We also calculated a Fisher Z-transformation concerning the correlations between clinical and electrophysiological parameters.
Results
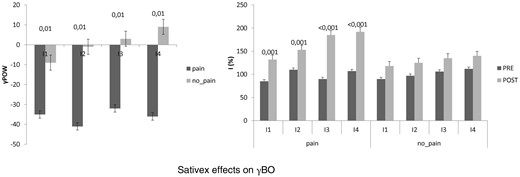
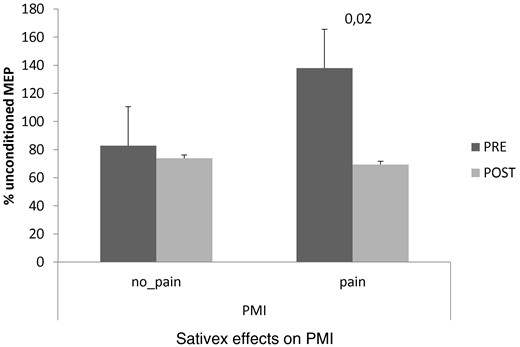
At baseline, we did not find any significant difference between pain-MS and no pain-MS groups concerning EDSS, NRS, and MSQoL ( Figure 1 ), as well as MEP amplitude, RMT, RIII thresholds ( Figure 2 ), and ET ( Figure 3 ) (the last two were significantly lower than our normative data: 18 ± 2 mA and 0.5 ± 0.25 J). Instead, VAS score was significantly higher in pain-MS than in no pain-MS patients ( P < 0.001). In addition, pain-MS group showed a strong reduction of γBO ( Figure 3 ) and PMI increase ( Figure 4 ) in comparison to no pain-MS. More in detail, the γBO synchronization did not correspond to the I ratio in pain-MS in comparison to no pain-MS ( Figure 3 ), in whom such correlation was present. An increase in N2P2 LEP latency and a reduction in amplitude were detected in both the groups, as compared with our laboratory age-matched LEP normative values ( Figure 5 ). In particular, pain-MS showed more delayed latencies than no pain-MS, whereas amplitudes were not significantly different.
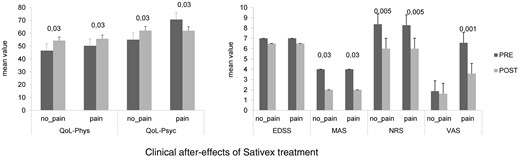
Shows the clinical effects after 4 weeks of Sativex administration (POST) in both pain and no pain-MS patients. NRS and MAS significantly decreased (* P < 0.05) in both groups, whereas VAS improved only in pain MS patients. EDSS: expanded disability status scale; MAS: modified Ashworth scale; VAS: visual analog scale; NRS: numerical rating scale. Values are reported as percent variation of the unconditioned value at baseline (PRE).
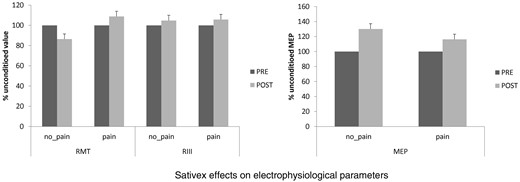
Shows that Sativex had no significant effects on resting motor threshold (RMT), motor evoked potential (MEP) amplitude, and RIII threshold in both pain and no pain-MS patients. Values are reported as percent variation of the unconditioned value at baseline (PRE).
Shows the gamma-band oscillations (γ-BO) at PRE (left panel) and the perceived pain rating (I) while increasing the energy of laser stimulation (right panel). Pain MS patients showed at PRE a more variable γ-BO than no pain-MS. Monthly Sativex administration restored the I-γ-BO relation, and increased it in no pain-MS. Values are reported as percent value.
Pain MS patients showed at baseline (PRE) a low PMI in comparison to no pain-MS individuals. The Sativex intake restored PMI strength in pain MS patients. Values are reported as percent variation of the unconditioned MEP at PRE.
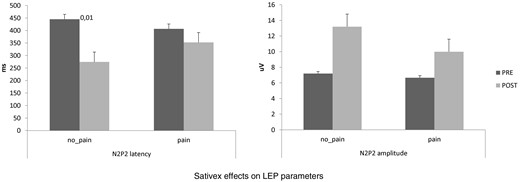
Reports the Sativex effects on laser-evoked potentials (LEP) latency and amplitude. We observed a reduction of LEP latency only in no pain-MS group. Values are reported as mean absolute values.
After 1 monthof Sativex intake, the most reported side-effects in the whole sample were dizziness, dry mouth, nausea, and weakness. No significant changes were observed in blood pressure, weight, temperature, and blood tests.
Concerning the clinical effects of Sativex medication, our patients showed a significant decrease in the subjective and objective spasticity parameters and the VAS score (only in pain-MS patients). MSQoL significantly increased in both groups ( Figure 1 ). Concerning electrophysiological data, no significant changes were found in RMT%, MEP amplitude, and RIII ( Figure 2 ). PMI was significantly reduced only in pain-MS ( Figure 3 ). The γ-band power was significantly increased after Sativex administration only in Cz within pain-MS ( Figure 4 ). Moreover, pain-MS patients showed a restored correlation among I and γBO ( Figure 4 ). LEP latency was slightly decreased only in no pain-MS ( Figure 5 ). Finally, correlation analysis in pain-MS patients evidenced a negative association between PMI/γ-band power, γ-band power/VAS, and VAS/MSQoL, and a positive one in PMI/VAS ( Table 2 ).
resumes the significant clinical-electrophysiological correlations within pain-MS patients after Sativex intake clinical-electrophysiological. We observed that the higher the γBO, the lower the PMI% and the VAS score. Moreover, we found that the lower the VAS score and the PMI%, the higher the MSQoL.
. | r . | P . | |
|---|---|---|---|
| PMI | γBO | –0.8 | 0.005 |
| VAS | γBO | –0.7 | 0.03 |
| MSQoL | –0.8 | 0.005 | |
| PMI | 0.9 | <0.001 | |
. | r . | P . | |
|---|---|---|---|
| PMI | γBO | –0.8 | 0.005 |
| VAS | γBO | –0.7 | 0.03 |
| MSQoL | –0.8 | 0.005 | |
| PMI | 0.9 | <0.001 | |
resumes the significant clinical-electrophysiological correlations within pain-MS patients after Sativex intake clinical-electrophysiological. We observed that the higher the γBO, the lower the PMI% and the VAS score. Moreover, we found that the lower the VAS score and the PMI%, the higher the MSQoL.
. | r . | P . | |
|---|---|---|---|
| PMI | γBO | –0.8 | 0.005 |
| VAS | γBO | –0.7 | 0.03 |
| MSQoL | –0.8 | 0.005 | |
| PMI | 0.9 | <0.001 | |
. | r . | P . | |
|---|---|---|---|
| PMI | γBO | –0.8 | 0.005 |
| VAS | γBO | –0.7 | 0.03 |
| MSQoL | –0.8 | 0.005 | |
| PMI | 0.9 | <0.001 | |
Discussion
Central neuropathic pain can be defined as “the pain initiated or caused by a primary lesion or dysfunction in the central nervous system” [ 38 ]. Central neuropathic pain occurs in about 70% of MS patients [ 39 ]. To date, its pathophysiology remains still partially understood, although its central origin has been called into question. Therefore, providing an adequate pain assessment and relief can be very challenging in MS patients.
Growing evidence suggests that cannabinoids; for example, Sativex, may be useful in the treatment of acute and chronic pain in many diseases, including MS, although recent studies have shown conflicting results [ 6 , 7 , 11–14 , 40 ]. Anyway, the role of Sativex in pain relief is not surprising, as CB1 receptors are located in the structures modulating nociception [ 41 ]. In addition, it has been shown that Sativex could act on the α3-subunit of glycine receptor (a non CB-receptor), which is thought to play an important role in the anti-nociceptive process [ 42 , 43 ]. Moreover, a direct interaction between CBD and S296 amino acid in the third trans-membrane domain of purified α3 GlyR [ 43 ] has been also hypothesized. Indeed, our data support the effectiveness of Sativex in pain relief in MS patients, as 1 month of drug intake successfully reduced the pain rating and improved their quality of life. We may also argue that Sativex could have boosted the cortical pain gating mechanisms, as the clinical pain relief was paralleled by an activation of sensory-motor areas, as suggested by centro-parietal γ-band power potentiation and PMI strengthening. Both these parameters are thought to be linked to the cortical anti-nociceptive phenomena and the subjective pain perception [ 44–46 ]. In healthy individuals, the higher the evoked γ-band power at cortical level is (that correlates with the subjective pain perception and, at the same time, the cortical anti-nociceptive phenomena), the greater the pain rating (expressed as I ratio, i.e., the pain rating corresponding to the energy of laser stimulation used). At baseline, such correlation was absent in our pain-MS group, suggesting an abnormal pain perception that does not completely depend on nociceptive pathway abnormalities. The altered pain perception in pain-MS patients is further suggested by the PMI dysfunction. After 1 month of Sativex intake, the I/γ-band power correlation and the PMI were restored in pain-MS, and further potentiated in no pain-MS group. Notably, such ameliorations were correlated to MSQoL and VAS improvement, thus further suggesting that Sativex may carry out its pain-relieving effects through a cortical pain-gating modulation; this, in turn, may be also related to the increase of the inhibitory tone at cortical level after Sativex administration, as previously shown [ 47 ].
Therefore, Sativex may restore cortical pain gating mechanisms, probably through a modulation of sensory-motor integration concerning painful stimulation (as suggested by the correlations among PMI, I/γ-band power, and clinical assessment).
As RIII was unmodified by Sativex administration, we were unable to demonstrate its effect at spinal cord level, by considering such parameter threshold. Hence, such issue needs to be clarified with further electrophysiological investigation.
Interestingly, Sativex reduced LEP latency only in the no pain-MS group. A LEP-latency increase in MS patients has been recently reported as expression of a demyelination damage of the spino-thalamic pathway, which produces multiple conduction blocks [ 48 ]. Thus, we may argue that pain-MS patients probably had a more severe functional damage of nociceptive pathways in comparison to no pain-MS. This could be the reason why Sativex was ineffective on LEP parameters in pain-MS patients. Moreover, it is hypothesizable that, when the nociceptive pathways are not severely impaired, Sativex is able to induce an amelioration of the conduction processes, as previously suggested in animal models [ 49 ]. However, such hypotheses need to be further investigated.
The main limitation of our study, beyond the small sample size, is that we cannot exclude the possibility of a placebo effect. However, our data are in line with previous findings including a control-group, and it is therefore hypothesizable that the Sativex treatment after-effects could be attributed to the drug itself. In addition, the patient sample was not so homogeneous, as: 1) some pain-MS subjects complained of intermittent neuropathic pain (although either the acute or the chronic manifestations of pain-related symptoms and signs may be common among different conditions and different pathophysiological mechanisms, thus being part of a clinical continuum) [ 48 , 50 ]; and 2) some no pain-MS patients complained of burning pain, although this was non-significant as suggested by the low VAS and DN4 scores (both below the cut-off significance, i.e., ≥4).
In conclusion, our data suggest that Sativex may be effective in improving MS-related neuropathic pain, at least in the short-term, maybe through its action on specific cortical pathways although an effect of Sativex at spinal/peripheral level should be assessed in future studies.
Funding sources: None.
Conflicts of interest: The author have none to report.
References
Author notes
Authors Margherita Russo and Antonino Naro contributed equally to this work.